66. Hyposthenuria, asthenuria, osmotic diuresis
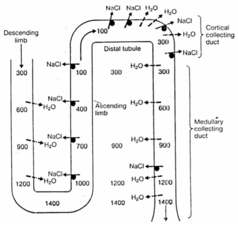
The cortico-medullary gradient
First, watch this video to understand the countercurrent system of the loop of Henle.
The osmolarity of the interstitium increases toward the medulla – we have a cortico-medullary osmotic gradient in the interstitium between the loops of Henle and the collecting duct. This osmotic gradient is essential for the kidneys ability to dilute and concentrate the urine. Let’s see why.
As explained in the video is the interstitial cortico-medullary gradient established because sodium is pumped out without water in the ascending limb, which increases the osmolarity of the interstitium but decreases the osmolarity of the filtrate. However, in the descending limb will water passively flow out from the filtrate into the interstitium because the osmolarity in the interstitium is much higher than in the filtrate.
When the filtrate now reaches the collecting tubule will the filtrate have much lower osmolarity than the interstitium surrounding the collecting tubule, which means that water will flow out from the filtrate and into the interstitium. Because the osmolarity of the interstitium increases toward the medulla will more and more water leave the filtrate as the filtrate travels toward the renal pelvis, concentrating the urine.
However, water doesn’t flow freely from the collecting duct and into the interstitium; it only does so through aquaporin channels. These aquaporin channels are only present when there’s ADH present, because ADH causes the collecting tubule cells to incorporate aquaporin channels into their filtrate-facing surface.
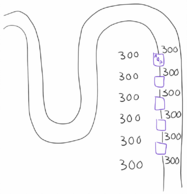
In other words are both ADH and the cortico-medullary gradient necessary for concentrating urine. If there was no cortico-medullary osmotic gradient will there be no force that forces water out from the filtrate and into the interstitium (water transport through aquaporins is passive and not active, after all). However, if the gradient is present but no ADH is present will there be no aquaporins to allow water to leave the filtrate (and there is diabetes insipidus). In both cases will urine be severely diluted.
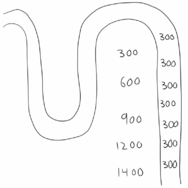
The figures above show the two extreme situations (absolutely no gradient and absolutely no aquaporins), but in real life there will mostly be some gradient and some aquaporins.
Hyposthenuria
So ADH’s ability to concentrate the urine depends on the cortico-medullary gradient. When the gradient is normal will the level of ADH regulate the urine’s specific gravity between 1.001 – 1.035 depending on whether the body needs to retain or lose water. When the gradient is smaller than normal is ADH’s ability to regulate the specific gravity also reduced. When the gradient is small can ADH only regulate the specific gravity for example between 1.004 – 1.020 (this can vary, as we’ll see) instead of the normal 1.001 – 1.035.
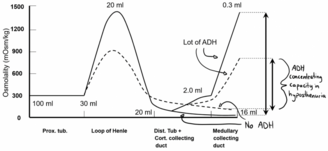
This is what’s known as hyposthenuria. Not that the specific gravity is necessarily low, but that ADH’s capacity to regulate the specific gravity is reduced.
Asthenuria
Asthenuria or isosthenuria is the complete loss of concentrating and diluting ability, due to there being no concentration gradient at all. The specific gravity of the urine will be the same as the specific gravity of the filtrate (so no dilution or concentration has occurred). It occurs in the end-stage of kidney failure.
The osmolarity of different fluids is summarized in the table below.
| Fluid | Osmolarity (in mOsm/kg) | Specific gravity |
|---|---|---|
| Ultrafiltrate (filtrate right after glomerulus) | 280 – 300 | 1.010 – 1.012 |
| Concentrated filtrate (at loop of Henle) | 1200 – 1300 | 1.030 – 1.035 |
| Urine (concentrated) | 1200 – 1300 | 1.030 – 1.035 |
| Urine (diluted) | 60 – 80 | 1.001 |
The range in which ADH can regulate the specific gravity of urine depends on what the specific gravity of the ultrafiltrate is. If the ultrafiltrate has a high specific gravity, for example as seen in diabetes where the specific gravity of the ultrafiltrate is 1.035, will the range be 1.024 – 1.040. The range is still narrow, but it’s shifted upwards.
This means that in hyposthenuria will the specific gravity of the urine be almost equal to the specific gravity of the ultrafiltrate.
Etiology
Hyposthenuria is caused by any condition that decreases the cortico-medullary osmotic gradient:
- Inhibition of Na+-reabsorption
- Diuretics
- Hypoxia
- Osmotic diuresis¸ where osmotically active substances (especially glucose) is in the filtrate, which causes less water to leave the filtrate in the descending loop
- Increased medullary perfusion will “wash out” the concentration gradients.
- Increased SNGFR causes the filtrate to flow faster through the tubules, which decreases the gradient
- Decreased GFR means that there is little salt to reabsorb, so the gradient decreases
- Low nephron density means that the gradient can’t be upheld
Consequences
Because the ultrafiltrate is normally relatively diluted (1.010) and in hyposthenuria is the ability to concentrate the urine lost, will urine in hyposthenuria also be diluted. The body loses its ability to regulate the fluid balance by conserving or excreting water based on the body’s hydration status. This means that in hyposthenuria is salt and water loss typical. This also means that a person with hyposthenuria is less protected against water-loads (increased water consumption) and salt-loads (increased salt consumption). Because of this may hypertonicity (the blood is hypertonic) and hypotonicity easily develop, depending on whether there is increased salt consumption or increased water consumption, respectively.
Hyposthenuria is also associated with alterations in urine volume¸ i.e. either oliguria or polyuria. In hyposthenuria is the urine output of each nephron always increased, but the total urine production obviously depends on how many nephrons are functional.
Potassium loss may also occur due to secondary hyperaldosteronism (RAAS activation) due to hypovolaemia due to polyuria. Aldosterone will cause increased potassium and H+ excretion.
Usually, when there is increased SNGFR (due to fewer functioning nephrons) must the tubules reabsorb a lot of the excess filtrate. However, if there is both increased SNGFR and hyposthenuria are the tubules be unable to reabsorb much of the filtrate, causing polyuric hyposthenuria.
The increased SNGFR eventually causes more and more nephrons to fail, so the polyuria can’t be maintained and is eventually replaced by oliguria, however hyposthenuria is still present. If there was no hyposthenuria would the tubules dilute the urine to compensate for the decreased water excretion, however in hyposthenuria are they unable to. This causes a rise in extracellular volume.
