4. Regulation of glycogen synthesis and degradation
Learning objectives
- Which protein regulates both glycogen synthase and glycogen phosphorylase?
- What is the function of casein kinase II?
- What is the net result of insulin on glycogen metabolism?
- What is the net result of glucagon and epinephrine on glycogen metabolism?
- How does insulin regulate glycogen synthase?
- How does insulin regulate glycogen phosphorylase?
- How does glucagon regulate glycogen synthase?
- How does glucagon regulate glycogen phosphorylase?
- How does epinephrine regulate glycogen synthase?
- How does epinephrine regulate glycogen phosphorylase in the liver?
- How does epinephrine regulate glycogen phosphorylase in the muscle?
- How is glucagon metabolism regulated by muscle contractions?
- How is glycogen synthase allosterically regulated?
- How is glycogen phosphorylase allosterically regulated?
Insulin, glycogen, and epinephrine
These hormones will be described in more detail in topics 31 – 33, but a small introduction here is in order.
Insulin is a hormone produced by beta cells in the Langerhans islets of the pancreas. The hormone is released in response to high levels of glucose in the blood. The role of the hormone is to reduce the blood glucose level. It achieves this by stimulate metabolic processes which reduce the blood glucose level, and to inhibit metabolic processes which increase the blood glucose level. Glycogen synthesis is one of the metabolic processes which reduce blood glucose level, while glycogen breakdown increases the blood glucose level.
Epinephrine (adrenaline) is a hormone released by the medulla of the adrenal gland. The hormone is released in response to acute stress and low levels of glucose in the blood. The role of the hormone is to prepare the body for a fight-or-flight reaction. A part of this preparation is to increase the blood glucose level, as the muscles needs a lot of energy either way. Epinephrine achieves this by stimulating metabolic processes which increase the blood glucose level, and by inhibiting metabolic processes which decrease the blood glucose level. Epinephrine stimulates glycogen breakdown and inhibits glycogen synthesis.
Glucagon is a hormone released by alpha cells in the Langerhans islets of the pancreas. Glucagon is basically the opposite hormone of insulin. It is secreted in response to low levels of glucose in the blood, and the role of the hormone is to increase the blood glucose level. It achieves this in a similar fashion as epinephrine.
While both epinephrine and glucagon have similar effects on biochemical pathways, they act on different tissues. Epinephrine acts on skeletal muscle and the liver, while glucagon acts only the liver. Insulin acts on both tissues.
Regulation of glycogen phosphorylase
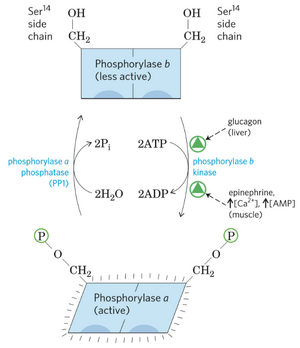
Glycogen phosphorylase has two forms, one active (a) form and one inactive (b) form. The enzyme is in its active form when it is phosphorylated and in its inactive form when dephosphorylated. Whether the enzyme glycogen phosphorylase is in the active or inactive form is determined by enzymes that either phosphorylate it or dephosphorylate it. The enzyme is also allosterically regulated.
Activators of phosphorylase kinase
Phosphorylase kinase is the enzyme responsible for phosphorylation, and therefore activation, of glycogen phosphorylase. When phosphorylase kinase is activated, it will phosphorylate and activate glycogen phosphorylase.
In the liver, glucagon activates a protein called protein kinase A (PKA). This protein phosphorylates and activates phosphorylase kinase, which activates glycogen phosphorylase.
In the muscle, epinephrine acts by the same pathway. It binds to beta receptors on skeletal muscle, which activates PKA. The rest occurs as for glucagon in the liver.
Epinephrine also acts on the liver, but by a different pathway. Epinephrine binds to alpha receptors on the liver, which activates another protein called protein kinase C (PKC). PKC increases the level of intracellular calcium, which activates another protein called calmodulin. Calmodulin activates phosphorylase kinase, which activates glycogen phosphorylase.
In muscle, phosphorylase kinase is activated by two other mechanisms. When muscle contracts, the level of ATP in the muscle decreases and the level of AMP increases. This stimulates a protein called AMP kinase (AMPK), which phosphorylates and activates phosphorylates kinase. When muscle contracts, intracellular calcium also increases. This stimulates calmodulin, which activates phosphorylase kinase.
Inactivators of phosphorylase kinase
Another enzyme called protein phosphatase 1 (PP1) dephosphorylates both phosphorylase kinase and glycogen phosphorylase, inactivating them both. PP1 is activated by insulin.
Allosteric regulation of phosphorylase kinase
In the liver, glycogen phosphorylase is also regulated allosterically by glucose, which inactivates it. This is a form of negative feedback.
Regulation of glycogen synthase
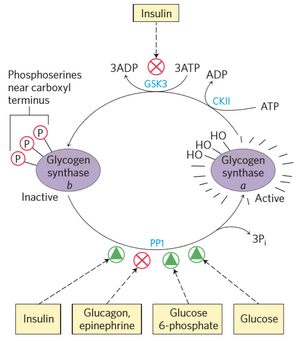
Glycogen synthase also has two forms, one active (a) and one inactive (b) form. In contrast to glycogen phosphorylase however, the active form is the dephosphorylated while the inactive form is phosphorylated. That means that phosphorylation of glycogen synthase will deactivate it, and dephosphorylating will activate it.
Activators of glycogen synthase
PP1 works on glycogen synthase as well as glycogen phosphorylase. By dephosphorylates glycogen synthase, PP1 activates it. PP1 is, in turn, activated by factors shown on the illustration to the right. PP1 is therefore the only regulator that directly regulates both glycogen synthase and glycogen phosphorylase.
Inactivators of glycogen synthase
The phosphorylation of glycogen synthase is regulated by multiple enzymes. The first one is glycogen synthase kinase 3 (GSK3), which phosphorylates glycogen synthase, deactivating it. However, GSK3 doesn’t work without another kinase, called casein kinase II (CKII). CKII primes glycogen synthase, which is necessary for GSK3 to work.
Insulin activates another protein kinase, called protein kinase B (PKB). PKB phosphorylates GSK3, inhibiting it. Insulin also activates PP1, which dephosphorylates glycogen synthase, activating it. Insulin therefore activates glycogen synthase, as it inhibits that which inhibits it.
AMP kinase, which is activated when levels of AMP in the cell is high, also phosphorylates and inactivates glycogen synthase. AMPK is further detailed in a later topic.
Allosteric regulation of glycogen synthase
Glucose and glucose 6-phosphate both allosterically activate glycogen synthase.
Summary
- Which protein regulates both glycogen synthase and glycogen phosphorylase?
- PP1
- What is the function of casein kinase II?
- CKII primes glycogen synthase, allowing GSK3 to act on it
- What is the net result of insulin on glycogen metabolism?
- Insulin stimulate glycogen synthesis and inhibits glycogen breakdown
- What is the net result of glucagon and epinephrine on glycogen metabolism?
- They stimulate glycogen breakdown and inhibit glycogen synthesis
- How does insulin regulate glycogen synthase?
- Insulin -> PKB, which inhibits GSK3, which inhibits glycogen synthase
- Insulin -> PP1, which activates glycogen synthase
- How does insulin regulate glycogen phosphorylase?
- Insulin -> PP1, which inhibits glycogen phosphorylase
- How does glucagon regulate glycogen synthase?
- Glucagon -> PKA, which inhibits glycogen synthase
- How does glucagon regulate glycogen phosphorylase?
- Glucagon -> PKA -> phosphorylase kinase -> glycogen phosphorylase
- How does epinephrine regulate glycogen synthase?
- Epinephrine -> PKA, which inhibits glycogen synthase
- How does epinephrine regulate glycogen phosphorylase in the liver?
- Epinephrine -> alpha receptor -> PKC -> increased intracellular calcium -> calmodulin -> phosphorylase kinase -> glycogen phosphorylase
- How does epinephrine regulate glycogen phosphorylase in the muscle?
- Epinephrine -> beta receptor -> PKA -> phosphorylase kinase -> glycogen phosphorylase
- How is glucagon metabolism regulated by muscle contractions?
- Muscle contractions -> decreased ATP, increased AMP -> AMPK -> phosphorylase kinase
- Muscle contractions -> increased intracellular calcium -> calmodulin -> phosphorylase kinase -> glycogen phosphorylase
- How is glycogen synthase allosterically regulated?
- Glucose and glucose 6-phosphate allosterically activate it
- How is glycogen phosphorylase allosterically regulated?
- Glucose allosterically inactivates it
