28. Intracellular proteolysis
Learning objectives
- Which factors influence the rate of degradation?
- How are intracellular proteins degraded in eukaryotes?
- How are endocytosed proteins degraded?
Intracellular protein degradation
Proteins in the cell are broken down constantly. To not waste resources, they should be recycled as much as possible.
The rate at which proteins are degraded varies greatly. Ornithine decarboxylase for example has a half-life of only 11 minutes. A protein called crystallin lasts as long as the organism itself. Transcription factors only live a few minutes before being degraded.
The rate of degradation depends highly on the amino acid residue on the N-terminal end of the protein. Amino acids like alanine and glycine increase the half-life, while amino acids like arginine and lysine decrease it.
The ubiquitin-proteasome system regulates gene transcription by degrading transcription factors. It also regulates the cell cycle and apoptosis (programmed cell death) by degrading related proteins.
Degradation in bacteria
Degradation in bacteria is performed by Lon proteases.
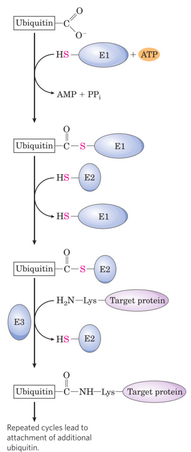
Degradation in eukaryotes
The protein to be broken down is first tagged with a protein called ubiquitin, a process that is catalysed by three enzymes, E1, E2 and E3. This process is called ubiquitination. The ubiquitin acts as a “flag”, signalling to the 26S proteasome that this protein is to be degraded. Proteins are usually tagged with many ubiquitins before they’re degraded; we say that they’re polyubiquitinated.
The ubiquitinated protein is then degraded by the 26S proteasome, a protein complex that degrade proteins by proteolysis. The proteasome is a barrel-shaped protein into which ubiquinated proteins enter and are degraded.
Proteolysis is the chemical breaking of peptide bonds. Complete proteolysis of a protein results in the amino acids the protein was built up of. These amino acids can then be broken down for energy or reused.
Degradation of endocytosed proteins
Endocytosed proteins are degraded by the endosome-lysosome system, in which the endosomes from the endocytosis are fused with lysosomes. Lysosomes have acidic pH, which is optimal for the hydrolytic enzymes it contains. These hydrolytic enzymes will proteolyse the endocytosed protein.
Summary
- Which factors influence the rate of degradation?
- The amino acid on the N-terminal end
- How are intracellular proteins degraded in eukaryotes?
- Proteins to be degraded are polyubiquinated
- Polyubiquinated are then degraded by the 26S proteasome
- How are endocytosed proteins degraded?
- Endocytosed proteins in vesicles fuse with lysosomes, which contain hydrolytic enzymes
