2. Molecular components of the immune systems
Antibodies
An antibody or immunoglobulin is a molecule found in the body that binds to antigens on pathogens. It does this to make it easier for the body and immune cells to get rid of the pathogens. The specific ways the immunoglobulin do this is written in more detail in topic 13.
The antibodies are the main component of the humoral (non-cellular) immune response, a part of the adaptive immune response. Antibodies are basically receptors; they are proteins that can bind to antigens. However, unlike other receptors, they can be found both on cell surfaces and in free form in the blood and in tissues. Antibodies bind to and attach themselves to antigens found on pathogens.
Antibodies are produced by B-cells, and each B-cell can produce many antibodies. However, all the antibodies produced by one specific B-cell will only recognize 1 specific antigen. The body produces many different B-cells though that each recognize different antigens, so that when a pathogen infects the body, at least one of the B-cells will produce antibodies against one of the antigens found on the pathogen. All antibodies produced by the same B-cell is said to be the same idiotype.
Strictly speaking, an antibody doesn’t bind to an antigen, it binds to a specific site on the antigen called the epitope. While an antigen is a molecule, the epitope is the specific part of the molecule that the antibody can bind to. One antigen can have many epitopes, so many antibodies can bind to the same antigen.
Isotypes
There are 5 isotypes of antibodies, each with slightly different functions. They are IgG, IgA, IgE, IgD and IgM. All the antibodies produced by one B-cell are the same isotype and idiotype.
Structure
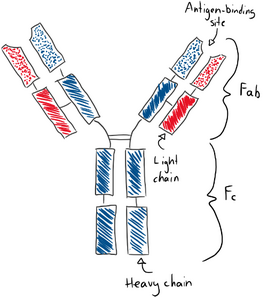
The antibodies have a very distinct structure that differs only slightly between each immunoglobulin isotype, and even less between immunoglobulin of the same isotypes. They are Y-shaped proteins that are symmetrical along the midline. They have two heavy-chains (in blue) and two light-chain (in red). They have 4 variable domains on their tip (dotted parts) and 8 – 10 constant domains (striped) based on their type. They have two antigen-binding sites (should actually be called epitope-binding sites) on its variable domains, so one antibody can bind to two antigens. The upper half of the antibody is the Fab (fragment antigen-binding) part, while the lower half is the Fc part.
The heavy chain can be of five types. If the heavy chain is α (alpha) type, then the immunoglobulin type is IgA. If the heavy chain is γ (gamma) type, then it’s type IgG. If it’s δ (delta), then it’s IgD. If it’s ε (epsilon), then it’s IgE. If it’s µ (mu), then it’s IgM. The light chain can also be two types, κ (kappa) or λ (lambda).
The different antibody subtypes have different properties. IgA is mostly found in mucosal surfaces, and exist in pairs connected to each other as a dimer. IgG in the most common form. It’s the only antibody that can cross the placenta. IgD is exclusively found bound to a B-cell and not soluble in the blood. IgE is important in allergy and its heavy chain has 4 domains instead of the usual 3. IgM exists only in clusters of 5 molecules bound together in pentamers. It’s part of the first immune response, before there is suficcient IgG. Its heavy chain is similar to IgEs.
The Fab part is involved with binding the antigen. After the antibody has bound the antigen, the Fc part becomes important. Cells can bind to the Fc part to phagocyte the antibody-antigen complex, start signal transduction within the cell, or recruit other systems.
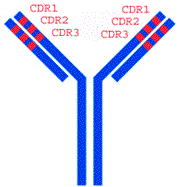
The constant domains of each antibody are similar, while their variable domains vary (hence the names). On each variable domain are three regions that vary wildly from antibody to antibody, called complementary-determining regions, or CDR. It’s ultimately these regions that determine what epitope the antibody will bind to, and how strongly it will bind.
B cell receptor
B-cells have receptors that can bind to and recognize antigens. This B cell receptor (BCR) is equal to an antibody in function and structure; it’s simply an antibody connected to its cell surface. The B cell receptor does however not work alone; it needs a co-receptor called CD79. Together, this B cell receptor complex can recognize an antigen, which initiates a signal transduction cascade inside that cell.
Both membrane-bound (the BCR) and secreted immunoglobulins are identical in any given B-cell, except that the membrane-bound immunoglobulin has a transmembrane and cytoplasmic part. When the B-cell wants to produce secreted immunoglobulins instead, they splice the exons that code for the transmembrane and cytoplasmic part out of the mRNA before translation. This causes the immunoglobulin to be translated without these two parts, which causes them to be secreted instead of bound to the membrane.
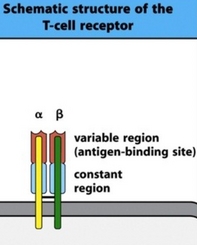
T cell receptor
Like the B-cell the T cell also has a T cell receptor (TCR). Its structure is simple. It has one alpha chain and one beta chain, and each of those chains have a variable region containing the antigen-binding site and one constant region. Like the B-cell receptor, it needs a coreceptor to transduct signals, called CD3. The T cell receptor recognizes, while the CD3 transduces the signal into the cell. The TCR cannot recognize free antigens anywhere, it can only recognize antigens that are presented by other cells, as we’ll see in the next topic.
